Overview
The MAPK Pathway
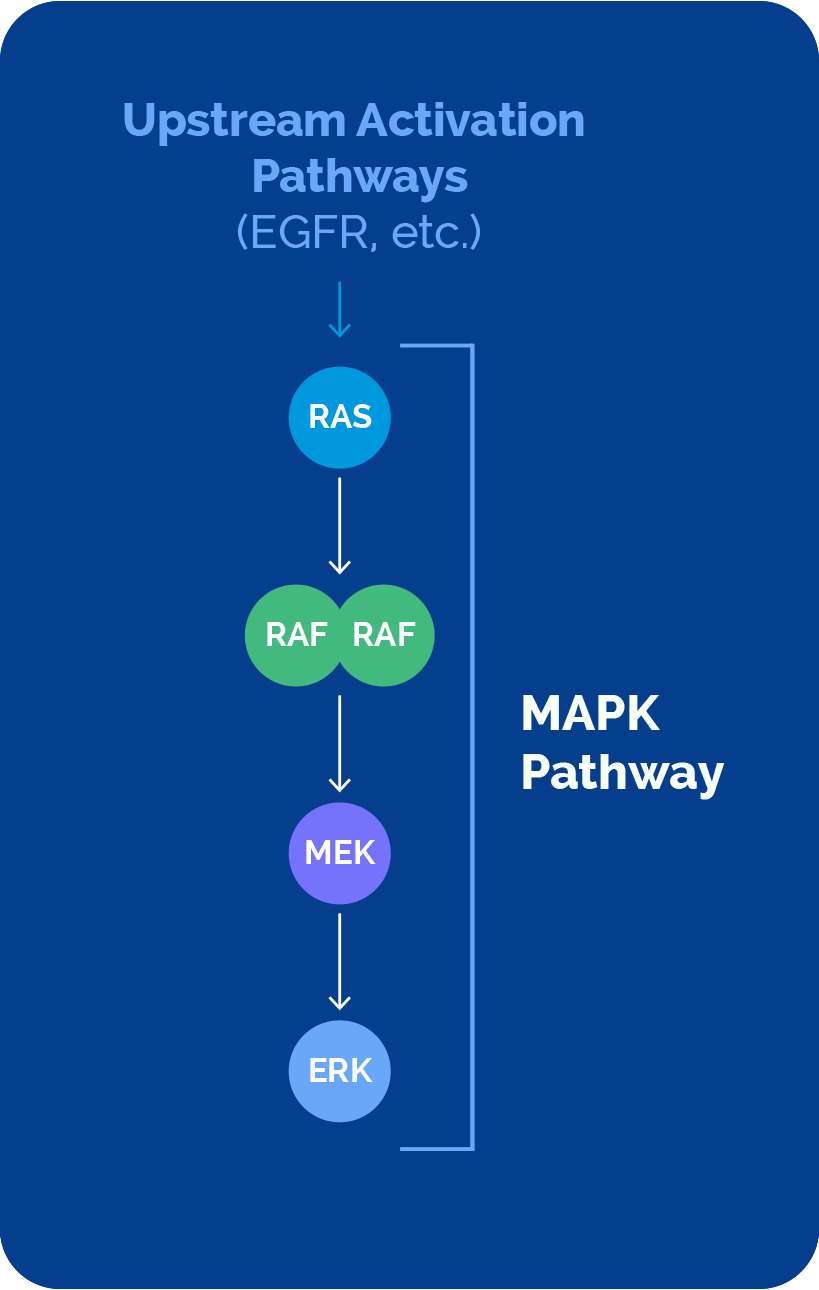
The MAPK pathway plays a major role in cancer development and progression, impacting millions of patients. This important pathway comprises a sequential cascade of four key signaling nodes – RAS, RAF, MEK and ERK.
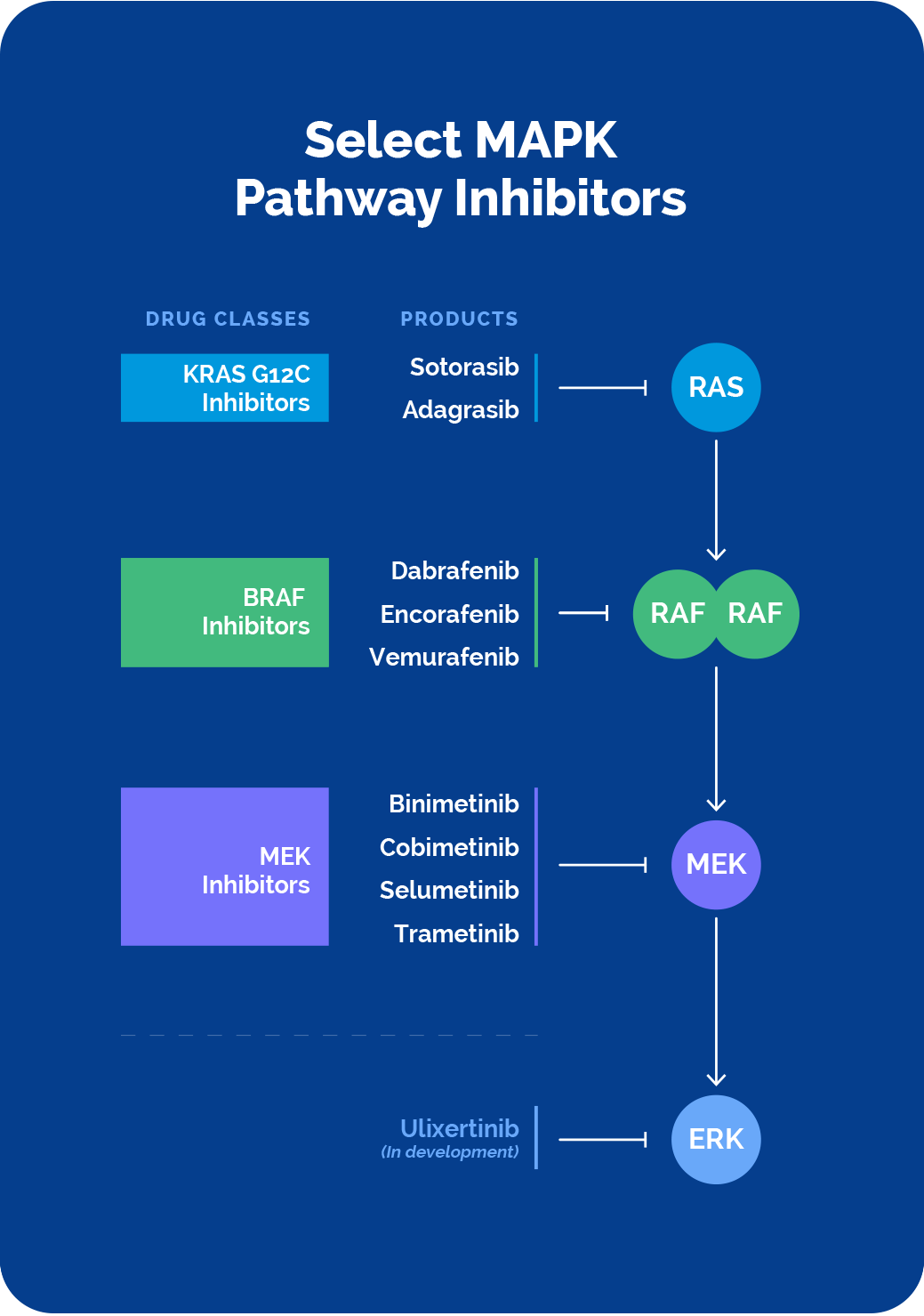
The last decade has seen the approval of precision therapies that target KRAS, BRAF and MEK. Despite this notable progress, challenges presented by the complex MAPK pathway biology as well as limitations of current MAPK pathway-targeted therapeutics continue to leave many patients without effective therapies for their cancer treatment.
Direct inhibition of ERK, the final node of the MAPK pathway, represents an important potential strategy to overcome these challenges and limitations.
Challenges & Unmet Need
MAPK pathway biology is notoriously complex
Complex Pathway Biology
The complexity of the MAPK pathway, with multiple nodes of activation and feedback mechanisms presents challenges in pursuing strategies for effective, durable treatment of MAPK pathway-driven cancers.
Direct or upstream pathway activation can drive cancer
Direct mutation of RAS, RAF and MEK proteins, or activation of the MAPK pathway via upstream mutations (i.e., EGFR), have been shown to contribute to disease.
Direct or upstream pathway inhibition has proven challenging
Direct intervention via the cancer-driving RAS, RAF and MEK proteins, or the upstream molecules that activate them, has been shown to be efficacious, but with limited durability. Both BRAF inhibitor reactivation of the MAPK pathway, known as paradoxical activation, as well as acquired mutations can lead to resistance.
There have been many different combinatorial strategies deployed, but these have been hampered by tolerability challenges and limited durability as well.
Unmet Needs in MAPK Pathway Treatment Landscape
Resistance mechanisms to direct and upstream inhibition of the pathway have limited durability and patient benefit
Approved BRAF, KRAS and MEK inhibitors continue to show limited durability, often due to resistance mechanisms that re-activate ERK signaling. Recent experience with many of the KRAS directed therapies have further supported this finding.
Tolerability and dosing challenges have made MAPK pathway combinations difficult to deploy in patients
Combinations of the various MAPK inhibitors with each other, or with the upstream inhibitors, is a viable approach to combat resistance, with some of these combinations approved. However, overlapping toxicities and subsequent suboptimal dosing to limit these toxicities, has made combinations with current therapies challenging to effectively deploy.
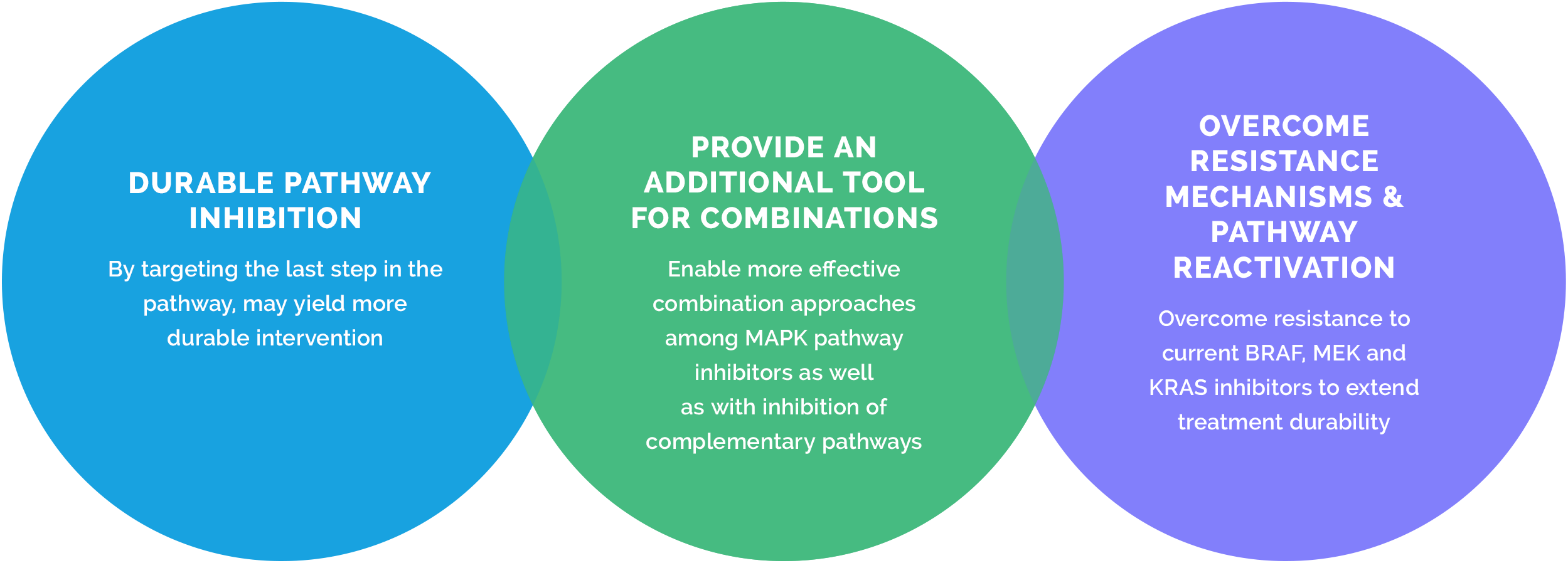
ERK inhibition is a critical and untapped strategy to address MAPK pathway cancers
Since ERK occupies the last node in the pathway, and many of the resistance mechanisms would still rely on ERK signaling to drive disease, a tolerable and effective ERK inhibitor represents an important strategy for the treatment of MAPK pathway-driven cancers.
The Opportunity & Potential of Ulixertinib to Help Patients
The complexity of MAPK pathway biology combined with the limitations of currently available therapeutics highlight the significant unmet needs for patients with MAPK pathway-mutated cancers.
At BVD, we are advancing ulixertinib (BVD-523), our highly selective, first-in-class ERK1/2 inhibitor, to harness the potential of ERK inhibition to bring effective, safe, and durable therapeutic options to more patients.

ERK Inhibition and Ulixertinib Potential
ERK represents a key target as the last node of the MAPK pathway
ERK activation is the final and necessary step in the MAPK signaling cascade. Direct inhibition of ERK could substitute for or be an important complement to inhibitors targeting these upstream MAPK pathway targets as well as auxiliary pathways, particularly to overcome resistance mechanisms.
Investigational ERK inhibitors have shown dosing and tolerability challenges
The ERK inhibitors that have been explored to date have shown a broad range of activities and tolerability profiles. The balance of both on-target and off-target inhibitory activities, and the subsequent tolerability challenges associated with them, is a critical component of development.

Ulixertinib’s differentiated, highly selective profile
Ulixertinib has demonstrated ERK selectivity (ERK1, 2 and 8), with limited off-target inhibition, giving it a potent and highly selective on-target profile that distinguishes it from other ERK inhibitors.
We have seen early evidence of ulixertinib’s differentiated highly selective profile translating to clinical activity and a favorable tolerability profile.
Ulixertinib’s potential for patients
Clinical Activity & Data
Ulixertinib’s class-leading clinical activity and data at-a-glance
Ulixertinib’s demonstrated efficacy and tolerability in the clinic to date, which we believe is attributable to its highly selective inhibition of ERK selectivity, distinguishes this molecule from other investigational ERK inhibitors.
Adult Clinical Studies & Patient Populations
- Over 400 patients treated – including completed 135- patient Phase 1b, expansion to two Phase 2 trials, and multiple investigator-initiated trials
- Being studied in tumor types requiring improved first-line and second-line+ treatment – glioma, histiocytosis, melanoma, pancreatic, colorectal
Observed Clinical Activity
- Observed evidence of clinical activity across broad range of tumor histologies and mutations (MAPK inhibitor-naïve and previously treated patients)
- Clinical activity in NRAS, BRAF V600, and non-V600-mutant solid-tumor malignancies
- Multiple examples of clinical benefit via compassionate use and Expanded Access Programs
Durability & Tolerability
- Demonstrated durable on-target inhibition and a favorable tolerability profile across multiple doses, including 150 mg BID, 300 mg BID, 450 mg BID, 600 mg BID
- Combination dose setting established with CDK4/6i and HCQ, ongoing with EGFRi and others
Adult & Pediatric Dosing
- Established recommended Phase 2 dose (RP2D) in adults (600 mg BID) and in pediatrics (260 mg/m2/dose PO BID)
- Established RP2D in combination with palbociclib and HCQ
- Demonstrated ability to dose ulixertinib intermittently without sacrificing efficacy in compassionate use setting
Upcoming Pediatric Phase 1 Clinical Study
- Expansion of the established pediatric RP2D in pediatric low-grade glioma (pLGG) planned for 2024
BID = twice daily; HCQ = hydroxychloroquine; QD = daily
*Established pediatric dose as part of the NCI MATCH trial
Ongoing Studies & Partnering
Ulixertinib Ongoing Development Program & Partnering Opportunities
We have built a clinical development program for ulixertinib that spans a range of cancer indications, MAPK pathway mutations, and combination approaches. Our development program has been informed by physician guidance and has evolved and expanded specifically to follow early, observed clinical signals. We are focused on indications with:
Documented frequency of MAPK pathway mutations
Few to no treatment options if patients develop resistance to approved MAPK pathway inhibitors
Demonstrated rationale to combine MAPK pathway inhibition with auxiliary pathway inhibition
Tumor Type
Monotherapy/
Combination Therapy
Preclinical
Phase 1
Phase 2
Phase 3
Tumor Type:
Histiocytosis
Monotherapy
Tumor Type:
Adult Low-Grade Glioma
Monotherapy
Tumor Type:
Pediatric Low-Grade Glioma
Monotherapy
Vinblastine
Tumor Type:
All Solid Tumor Cancers
(Expanded Access Program)
Monotherapy
I-O, Chemotherapy, BRAFi, EGFRi, CDK4/6i, and HCQ
Tumor Type:
Pancreatic, colorectal, esophageal, cholangiocarcinoma, gastric
Hydroxychloroquine (Autophagy inhibitor)
Tumor Type:
Melanoma
Palbociclib (CDK4/6 inhibitor)
Tumor Type:
Colorectal Cancer
EGFRi +/- BRAFi
Tumor Type:
Solid and Liquid Tumors
JAKi, RASi and RAFi*
I-O = Immuno-oncology; HCQ = Hydroxychloroquine
*Per our partner-centric corporate strategy, we are actively seeking early development partners to explore ulixertinib’s differentiated ERK1/2 inhibition profile in combination with MAPK pathway inhibitors and auxiliary pathway inhibitors where the underlying disease biology and limitations of current therapeutic strategies present the potential to address high unmet patient needs.
Our prioritized combination therapies of interest for early development include JAKi, RASi, and RAFi. However, given the broad potential of ERK1/2 inhibition in solid and liquid tumors, we are open to investigating additional combination therapy strategies.