Despite significant advances in oncology, some tumors remain too aggressive or difficult-to-reach to be addressed by existing therapies. We are advancing a unique modality – a cancer-fighting bacteria – to reach the difficult-to-access necrotic regions deep inside tumors, which are made up of dead or dying cells. The cancer-fighting bacteria are designed to attack and destroy tumors from the inside out. Necrosis is often associated with aggressively growing tumors, and recent research suggests that factors within the necrotic core may promote metastasis. Conventional therapies typically cannot reach these regions, representing a significant unmet need. We believe this therapeutic approach may support limb salvage for patients with tumors in their extremities, as well as tumor shrinkage or destruction in soft tissue sarcomas, bone and joint cancers, and potentially other cancers.
Why Target ‘Dead’
Cancer Cells?
As tumors enlarge, they outgrow their nutrient supply and, thus, their capacity to keep the entire tumor alive. Regions inside the tumor can become necrotic, or die, because they aren’t getting sufficient blood supply. However, a cancer-fighting presence in these regions is critical because not all cancer cells within these necrotic cores die, and they may contribute to cancer’s growth and progression. Indeed, recent findings from researchers at the Fred Hutchinson Cancer Center showed a direct link between tumor necrosis, circulating tumor cells, and cancer metastasis.

Overview
Program Overview
We are researching Clostridium novyi-NT (CNV-NT/BVD-550) – an attenuated form of the Clostridium novyi, or C. novyi, bacteria – as a modality to target the necrotic cores of tumors and unleash a powerful tumor-destroying attack.
C. novyi is a spore forming, anaerobic bacterium, meaning that it can only thrive in hypoxic regions (regions that lack oxygen) such as the necrotic core of a tumor. The C. novyi spore will only germinate, or turn into a live bacterium in hypoxic regions, otherwise it remains latent. The consequence of germination and growth of CNV-NT results in direct tumor destruction as well as activation of the host immune system.
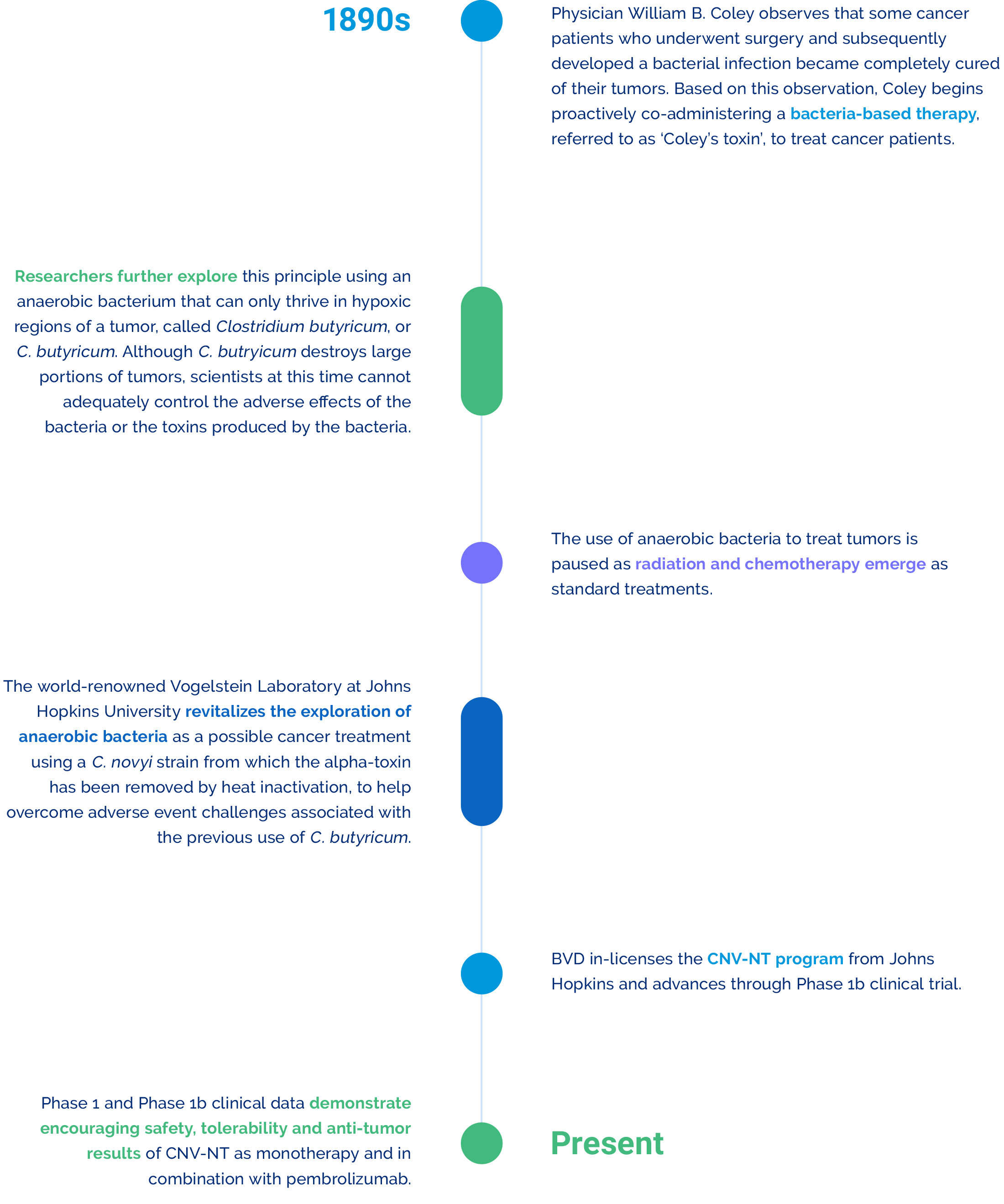
History & Rationale
Program History & Scientific Rationale
With our CNV-NT program, we are revitalizing the promising prospect of using bacteria as a cancer-fighting modality, which is rooted in scientific history and supported by strong scientific rationale.
Clinical Activity & Data
CNV-NT Clinical Activity
Since in-licensing the CNV-NT program, we have made important progress to better understand and elucidate its application in cancer, as well as explore the potential of intratumoral (iTu) administration in addition to intravenous (IV) administration.
Canine Clinical Studies
We conducted 11 canine clinical trials of CNV-NT in 225 companion dogs presenting with spontaneous tumors, evaluating both IV and iTu administration and saw dramatic anti-tumor results across the trials.
Phase 1 Monotherapy Clinical Study
In collaboration with MD Anderson Cancer Center, we conducted a Phase 1 study (NCT01924689) that evaluated iTu injection of CNV-NT as a monotherapy in cancer patients, which demonstrated encouraging safety, tolerability, and anti-tumor results.
The researchers enrolled 24 patients with treatment-refractory solid tumors, including 15 patients with sarcoma, seven patients with diverse carcinoma, and two patients with melanoma. Of the 22 evaluable patients, 21 had stable disease as measured by RECIST for the injected lesion, with tumor shrinkage of greater than 10 percent observed in 23 percent of patients. When both injected and uninjected lesions were included, the stable disease rate was 86 percent. Researchers also saw improved tumor-specific immune responses through the increased secretion of T cell cytokines and the increased presence of tumor-infiltrating lymphocytes in injected tumors.
Phase 1b Combination Clinical Study
The innate immune response elicited by CNV-NT in the Phase 1 monotherapy trial suggests this therapeutic modality may be synergistic with checkpoint inhibition. To that end, BVD and The University of Texas MD Anderson Cancer Center subsequently initiated a Phase 1 study of CNV-NT iTu in combination with pembrolizumab (NCT03435952) to evaluate whether pairing CNV-NT with an immunotherapy could amplify and enhance the durability of its cancer-fighting attack. The findings were published in Clinical Cancer Research, a journal of the American Association for Cancer Research.
A total of 16 patients with advanced solid tumors were enrolled in the Phase 1b study. The combination therapy was generally well tolerated, with one grade 3 dose-limiting toxicity of abscess formation.
The Phase 1b study demonstrated an overall objective response rate (ORR) of 25% (n=4) among patients with various advanced solid tumor malignancies, including nonkeratinizing undifferentiated nasopharyngeal squamous carcinoma, human papilloma virus–positive squamous cell carcinoma of the base of the tongue, vulvar melanoma, and chordoma (a rare cancer that originates in the bones of the spine or the skull). Among these patient responders, three partial responses and one complete response were observed, with a median duration of response of 10.93 months. Stable disease was observed in 69% of patients.