Overview
Program Overview
TEM8/ANTXR1: A Biomarker Across Multiple Solid Tumors
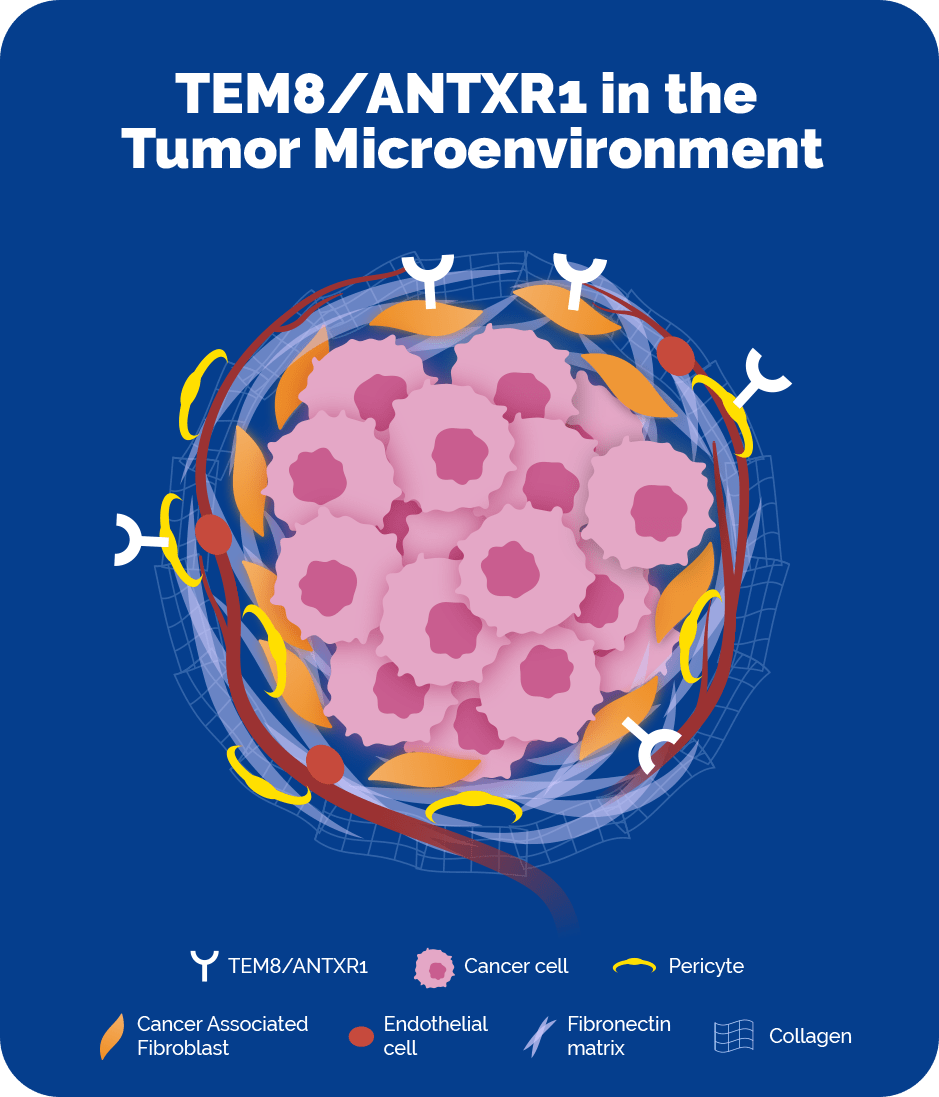
Tumor endothelial marker 8 (TEM8), also known as anthrax toxin receptor 1 (ANTXR1) is a surface protein that is highly expressed on cancer-associated fibroblasts (CAFs) and pericytes, which are both central players in the tumor microenvironment across multiple solid tumor cancers, including breast, colon, lung, and pancreatic cancers. TEM8/ANTXR1 shows limited expression on cells outside of the tumor microenvironment, making it a clear and precise beacon enabling anti-cancer payloads to target these key cells while minimizing the opportunity for off-target toxicity.
Importance of Targeting CAFs and Pericytes
CAFs and pericytes are believed to be important targets for tumor-fighting therapies because they facilitate cancer progression in a number of ways. Research indicates that they support tumor cell growth, and mediate tumor-promoting inflammation and immunosuppression. Because of the activity of CAFs and pericytes in the tumor microenvironment, tumors are able to evade current therapeutic approaches and/or develop resistance mechanisms, resulting in cancer progression and metastases. TEM8/ANTXR1 has the potential to directly target the CAFs and pericytes as well as indirectly target the primary cancer cells via a bystander mechanism.
Potential Utility in Multiple Modalities
We believe our TEM8/ANTXR1-directed therapeutic antibodies could serve as a cornerstone across multiple therapeutic modalities. We are currently focused on examining TEM8/ANTXR1-directed antibody-drug conjugates (ADCs) to more fully understand this protein’s potential in the fight against cancer. By disrupting the tumor microenvironment, these next-generation ADCs may result in optimal cancer cell destruction either as monotherapy or in combination with other anti-cancer agents.

Pre-clinical Activity
Program History & Preclinical Activity
There have been approximately 160 publications on TEM8/ANTXR1 over the past 20 years, including research showcasing promising findings for TEM8/ANTXR1 as a relevant cancer biomarker. Nevertheless, to date, TEM8/ANTXR1 has largely remained unexplored therapeutically.
BVD’s TEM8/ANTXR1 research was conducted under a cooperative research and development agreement (CRADA) with the National Cancer Institute. We are currently generating preclinical data on TEM8/ANTXR1-directed ADCs and plan to proceed toward investigational new drug (IND)-enabling activities.
We have generated promising preclinical data in TEM8/ANTXR1-directed ADC tool constructs in multiple cancers, including ovarian, breast, lung, colon, and pancreatic cancer models. In these studies, our TEM8/ANTXR1-directed ADC constructs prolonged survival, blocked metastasis, and augmented the efficacy of conventional chemotherapy agents without evidence of toxicity. Building on this preclinical evidence, as we advance toward first-in-human clinical studies, we are investigating the optimal initial cancer indications and patient populations and a variety of ADC linker/payload combinations.

ADC Opportunity
TEM8/ANTXR1’s Opportunity in ADCs
TEM8/ANTXR1 has several key characteristics that make it an optimal ADC-directed target:

Predominantly expressed on cells in the tumor microenvironment, with low expression on normal, healthy cells

Demonstrates broad expression in the tumor microenvironment, enabling the flexibility to advance ADCs that can address multiple solid tumor indications

Unique biology may allow for different ADCs to be effective via different mechanisms
As we continue to advance TEM8/ANTXR1 biology in the translational setting, including an understanding of optimal cancer indications, and de-risk our TEM8/ANTXR1 therapeutic antibodies in the clinic via TEM8/ANTXR1-directed ADCs, we plan to build on this knowledge to pursue modalities that represent increasingly higher technology hurdles, including NK cell engagers and cellular therapies.
Educational Videos
The educational video series below highlights TEM8/ANTXR1 as an emerging tumor microenvironment target for ADCs. This therapeutic approach has the potential to address significant unmet needs across multiple types of cancer and bring hope for life to patients.
An Emerging Tumor Microenvironment-Targeted Antigen for ADCs
TEM8/ANTXR1 is a surface protein that is highly expressed on cancer-associated fibroblasts and pericytes, two cell types that are enriched in the tumor microenvironment of multiple tumor histologies and minimally expressed on normal healthy cells.
We’re harnessing TEM8/ANTXR1 as a powerful lamp post to precisely guide cytotoxic payloads into the tumor microenvironment.
CAFs and Pericytes as Targets for Clinical Intervention via ADCs
CAFs and pericytes are highly enriched in the tumor microenvironment of many different types of cancer, and play a key tumor-promoting role. TEM8/ANTXR1 is a protein that is highly expressed on CAFs and pericytes, with minimal expression on normal, healthy cells.
At BVD, we’re advancing TEM8/ANTXR1-directed ADCs to precisely deliver cytotoxic payloads to TEM8/ANTXR1-expressing CAFs and pericytes, with the potential to bring a new therapeutic approach to a broad spectrum of cancers.
Potential for TEM8/ANTXR1-targeting ADCs in the Clinical Setting
In several types of cancer, including lung, colon, breast, and pancreatic, the tumor microenvironment can comprise a majority of the cancer mass. We’ve shown that TEM8/ANTXR1 is highly expressed on cancer-associated fibroblasts and pericytes within the tumor microenvironment of these solid tumor types.
Targeting ADCs to TEM8/ANTXR1 has significant potential in the clinical setting, both as a monotherapy or in combination with other anti-cancer agents.
Current Status of BVD’s Development of TEM8/ANTXR1-directed ADCs
BVD has generated promising preclinical data with our TEM8/ANTXR1 ADC tool constructs in multiple cancers, including ovarian, breast, lung, colon and pancreatic cancer models.
Based on these findings, and the unique expression pattern of the TEM8/ANTXR1 antigen, we’re evaluating multiple linker and payload combinations to identify a lead candidate for IND-enabling studies.